Diagnostic Parasitology: Fecal Samples (Common Floatation Solution for The Fecal Flotation Technique & Diagnostic Techniques)
DIAGNOSTIC
PARASITOLOGY: FECAL SAMPLES
(Common Floatation Solution for The
Fecal Flotation Technique & Diagnostic Techniques)
Diagnostic of parasitic infection
depends on several factors, such as collection of samples, transport of samples
to laboratory, and method of laboratory evaluation.
- Feces must be fresh for accurate result. As feces age, a diagnosis is complicated because many parasite eggs develop and hatch into larvae. Contaminants such as free-living soil nematodes, fly larvae, mites, and other arthropods often invade feces and complicate a diagnosis.
- At least 10 g of fresh feces should be collected. If samples are more than two hours old, samples should be stored at 4 degrees C until examined. Many parasite stages can be stored at 4 degrees C for at least two months with minimal development
- For routine shipment to laboratory, samples can be cooled to 4 degrees C and then packed with ice or other coolant (blue ice) for shipment via any of the 24- to 48-hour transport services.
- Fecal samples are best stored and sent in whirl-pak bags, small plastic sandwich bags, plastic containers, disposables laboratory gloves turned inside out, or rectal palpation gloves turned inside out.
- All samples should be clearly labelled with a black indelible marker with the number of the animal, date, and the person responsible for the sample.
Important comparative factors in the
fecal flotation technique are:
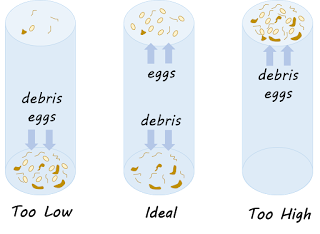
- The specific gravity of flotation solution. A specific gravity that is too low will not float many stages, whereas a solution with a specific gravity that is too high will cause plasmolysis, osmosis, or rupture of the stage, making diagnosis difficult. Also, as the specific gravity is increased, excessive debris also floats, which decreases the efficiency of the test. Most parasitic stages float efficiently at specific gravity of 1.2 to 1.3. we can use sugar solution (specific gravity = 1.27).
- The viscosity or type of solution used.
- The rate of plasmolysis caused by the solution.
Effect of specific gravity on egg flotation (Foreyt, 2001)
COMMON
FLOATATION SOLUTION FOR THE FECAL FLOTATION TECHNIQUE
Sugar
(specific gravity = 1.27)
Granulated
sugar (sucrose)
|
454
g
|
Tap
water
|
355
ml
|
Dissolve
sugar in hot tap water directly, or add sugar to hot water over a low heat
and stir. Approximately 2 ml of 37% formaldehyde or phenol crystals can be
added to deter growth of mold.
|
|
- Sugar has a distinct advantage over salt solution in that less plasmolysis and distortion occur in the eggs and oocysts. When salt solutions are used, egg distortion usually occurs in few hours, and the slides have a tendency to crystallize and dry out very quickly.
- Fecal-flotation slide preparations from sugar solution can be kept at 4 degrees C for at least 24 hours, and often several weeks to months, with minimum of distortion of eggs. These slide can be used as next-day reference and teaching slides.
- Some parasite eggs, such as the salmon poisoning fluke, Nanophyetus salmincola, float better in sugar than salt solution. In salt solution, the eggs often do not float and are often missed during examination.
- Disadvantage of sugar solution are the sugar can be messy and sticky, and sugar attracts flies and other arthropods. To increase the shelf life of fecal-flotation slide, one can put fingernail polish or quick-drying glue around the cover-slip. Freezing the prepared slide is often effective for preserving the material for many month or years.
Sodium
chloride (specific gravity = 1.18-1.2)
Saturated
NaCl or NaCl
|
400
g
|
Tap
water
|
1000
ml
|
Magnesium
sulfate (specific gravity = 1.2)
MgSO4
|
400
g
|
Tap
water
|
1000
ml
|
Zinc
sulfate (specific gravity = 1.18)
Zinc
sulfate
|
371
g
|
Tap
water
|
1000
ml
|
Sodium
nitrate (specific gravity = 1.18-1.2)
Sodium
nitrate
|
400
g
|
Tap
water
|
1000
ml
|
DIAGNOSTIC
TECHNIQUES
Modified
double centrifugation technique
1
|
Mix
1 g of feces in 10-12 ml water in a beaker and stir until feces are in
suspension.
|
2
|
Pour
the mixture through a tea strainer into another beaker. Press the material in
the strainer with a spatula and discard the material in the strainer.
|
3
|
Pour
the contents into a 15 ml centrifuge tube and fill to the top with water.
|
4
|
Centrifuge
the tubes at 1,500 rpm for 5-10 minutes.
|
5
|
Decant
the tube and then fill one half full of flotation solution. Stir the sediment
with a wooden applicator stick and then fill the tube almost to the top with
flotation solution.
|
6
|
Place
the tube in the centrifuge and with a dropper add flotation solution to the tube
so the solution is about level with the top of the tube. Place a 22-mm2
coverslip on the top of the tube and in contact with the sugar solution.
|
7
|
Centrifuge
at 1,500 rpm for 5-10 minutes. The coverslip will not fall if the centrifuge
has free-swinging trunnions that swing out to the horizontal position.
|
8
|
Remove
coverslip by lifting straight upward, and place on a glass slide. All
parasite stages that floated should be in the drop under the coverslip.
|
9
|
Examine
the slide under x100 (x10 ocular and x10 objective) or higher magnification
and observe all parasite stages present.
|
Note:
|
The
centrifuge must have free-swinging buckets. If the centrifuge has a fixed
bucket position, the coverslip will fall off. Fecal flotation can also be
done without centrifugation by allowing samples in step 4 to set for 20 minutes
and in step 7 to set for 30 minutes. This method is not as effective as
centrifugation, but results are usually reliable.
|
Ether-formalin
sedimentation technique
This technique is good for
detection of trematode eggs.
1
|
Mix
1 g of feces in 15 ml of water, strain the mixture, and pour into a 15-ml
centrifuge tube.
|
2
|
Centrifuge
at 1,000 rpm for 1-2 minutes.
|
3
|
Decant
supernatant, add fresh water, and centrifuge again for 1-2 minutes.
|
4
|
Decant,
add 10 ml of 10% formalin, and let stand for 10 minutes.
|
5
|
Add
3 ml of ether, apply a stopper, and shake the content vigorously. Centrifuge
the mixture for 2 minutes.
|
6
|
Remove
the debris on the top of the tube with a cotton-tipped swab. Decant the rest
of the fluid.
|
7
|
Collect
the sediment with a pipette, place it on a microscope slide, and examine it
microscopically for parasite eggs.
|
Fecal
sedimentation technique for Fasciola
hepatica and some other fluke eggs
1
|
Mix
5 g feces in 200 ml water in a beaker.
|
2
|
Pour
the mixture through a tea strainer and discard the material in the strainer.
|
3
|
After
10 minutes, decant approximately 70% of the supernatant and refill the beaker
with fresh water.
|
4
|
Repeat
step 3 for three to five times until the supernatant is clear.
|
5
|
Pour
off 90% of supernatant and pour the sediment into a Petri dish.
|
6
|
Examine
the sediment under a dissecting microscope (x20-x30) or scanning objective
(x4) of microscope (total magnification = x40) for large, yellow, operculated
egg of Fasciola hepatica.
|
Baermann
technique for lungworm larvae isolation
1
|
Place
warm water (approximately 25 degrees C) into a glass funnel that has a
stopcock or clamp on a rubber hose in the end of the funnel.
|
2
|
Take
5 g (or more) feces, wrap the feces in two layers of gauze and place the
feces in te water in the funnel.
|
3
|
After
8 hours, withdraw the bottom 10-15 ml of fluid from the funnels into a 15-ml
centrifuge tube.
|
4
|
Centrifuge
the tube for 5 minutes at 1,500 rpm.
|
5
|
With
a pipette, withdraw the bottom 2-3 drops from the centrifuge tube and
transfer to microscope slide.
|
6
|
Add
a coverslip and look for larvae under the microscope.
|
Modified McMaster technique for parasite eggs
This is dilution method of
estimating eggs present in fecal samples.
1
|
Mix
3 g feces in 15 ml water and pour through a tea strainer.
|
2
|
Pour
strainer material into a 15-ml centrifuge tube and centrifuge at 1,500 rpm
for 2 minutes.
|
3
|
Mix
the sediment in 10 ml of flotation solution and pour into a beaker, add
additional 32 ml flotation solution.
|
4
|
With
a pipette, transfer suspension to a McMaster counting chamber and fill both
chambers.
|
5
|
Transfer
the slide to a microscope and count all eggs inside the ruled squares.
|
6
|
Multiply
the number of eggs in both chambers by 50 for the total number of eggs per
gram of feces.
|
Note:
|
This
technique is not very accurate for samples with small numbers of eggs.
|
Direct
smear
This technique is used primarily
for diagnosis of Giardia.
It is very inefficient technique for diagnosis
of other parasite infections.
1
|
Mix
a fecal sample the size of the head of a match (1-2 mm3) with a drop of water or saline on a microscope
slide.
|
2
|
Mix
the drop with a circular motion until the specimen is approximately 1 x 1 cm.
|
3
|
Add
a coverslip and examine under the microscope.
|
4
|
If
large particles are present under the coverslip, remove the particles or
start with a new samples.
|
Reference:
Foreyt, W.J., 2001. Veterinary
Parasitology, reference Manual. 5th edition. Blackwell Publishing.
Iowa State University Press.



Komentar
Posting Komentar